What Covid 19 Antibody Tests Are Fda Approved
How useful or accurate are those tests. Singapores Covid-19 neutralising antibody test kit first to get US FDA approval.

Covid 19 Antibody Rapid Test Kit Coronavirus Igm Igg Antibody Test
Doctors say COVID-19 antibody tests arent FDA approved should be taken with caution Thousands of people are paying out of pocket to take an antibody test at private labs in the valley.
What covid 19 antibody tests are fda approved. 20 APRIL 2020. Food and Drug Administration FDA recommends that clinical laboratories and health care providers stop using COVID-19 antibody tests that are listed on FDAs removed test list. FDA Authorizes First Test that Detects Neutralizing Antibodies from Recent or Prior SARS-CoV-2 Infection.
There are no currently available FDA approved COVID-19 test kits in the Philippines that differentiate the antibody protection gained from natural COVID-19 infection and the immunity from vaccination. FDA Authorizes 2 Over-The-Counter Coronavirus Tests. Food and Drug Administration FDA has approved for emergency use.
FDA News Release. The FDAs Experience with Covid-19 Antibody Tests Early in the pandemic the FDA recognized that ensuring access to antibody tests could advance understanding of. Text Version of Infographic.
HOUSTON The Regeneron cocktail is probably best known as the monoclonal antibody treatment given to President Donald Trump after he contracted COVID-19. Celia Carlos informed FDA Director General Rolando Enrique Domingo that the Institute can now evaluate approved. FDA has granted emergency use approval to an antibody test with 100 accuracy developed by Roche The test is called Elecsys Anti-SARS-CoV-2 It uses IV blood draws and not finger-prick assays.
This page is updated daily by 500 am EST. The Research Institute for Tropical Medicine RITM is now ready to evaluate rapid antibody test kits for COVID-19 disease. Health Canada confirms that authorized COVID-19 tests are well supported by evidence indicating they will provide accurate and reliable results.
519 rows The FDA today announced it has issued an emergency use authorization EUA for the Symbiotica COVID-19 Self-Collected Antibody. But what about the tests the US. The FDA is committed to helping ensure the public has access to a wide variety of test.
COVID-19 Tests and Collection Kits Authorized by the FDA in 2020. In a letter received by the Food and Drug Administration FDA on 17 April 2018 RITM Director Dr. Orthos new quantitative COVID-19 IgG antibody test targets the S1 spike protein and is intended for use in identifying individuals with an adaptive immune response to SARS-CoV-2.
Eight cases on Mon Nov 9 By Timothy Goh The Straits Times Singapore. The FDA is reminding the public of the limitations of COVID-19 antibody or serology testing and providing additional recommendations about the use of antibody tests. In some validation studies of these tests like the one FDA is conducting in partnership with NIH CDC and BARDA the samples used in addition to.
Coronavirus Updates The Food and Drug Administration says Abbotts BinaxNOW test. So far the FDA has approved 12 antibody tests through the emergency use processincluding those from large manufacturers such as Roche Ortho and Abbottand most of them only in the last few. SARS-CoV-2 antibody often referred to as serology tests look for antibodies in a sample to determine if an individual has had a past infection with the virus that causes COVID-19.
The table below includes applications that are under evaluation. In a recent post we warned readers against rushing out to purchase one of the many COVID-19 antibody tests suddenly flooding the market noting that few of these tests have been independently validated and many are grossly inaccurate. Unauthorized tests may not produce accurate results leading to potential misdiagnosis.
These kits have varying specifications and indications independent from each other which are helpful in specific circumstances and settings. Since the beginning of the COVID-19 public health emergency the FDA has balanced the urgent need for access to diagnostic and antibody tests with providing a level of oversight that helps to. The FDA approved it back in November as an emergency use drug for mild to moderate COVID-19 cases or for those who are high-risk.
/cdn.vox-cdn.com/uploads/chorus_asset/file/19867086/1207418976.jpg.jpg)
Fda Authorizes First Antibody Based Test For Covid 19 The Verge
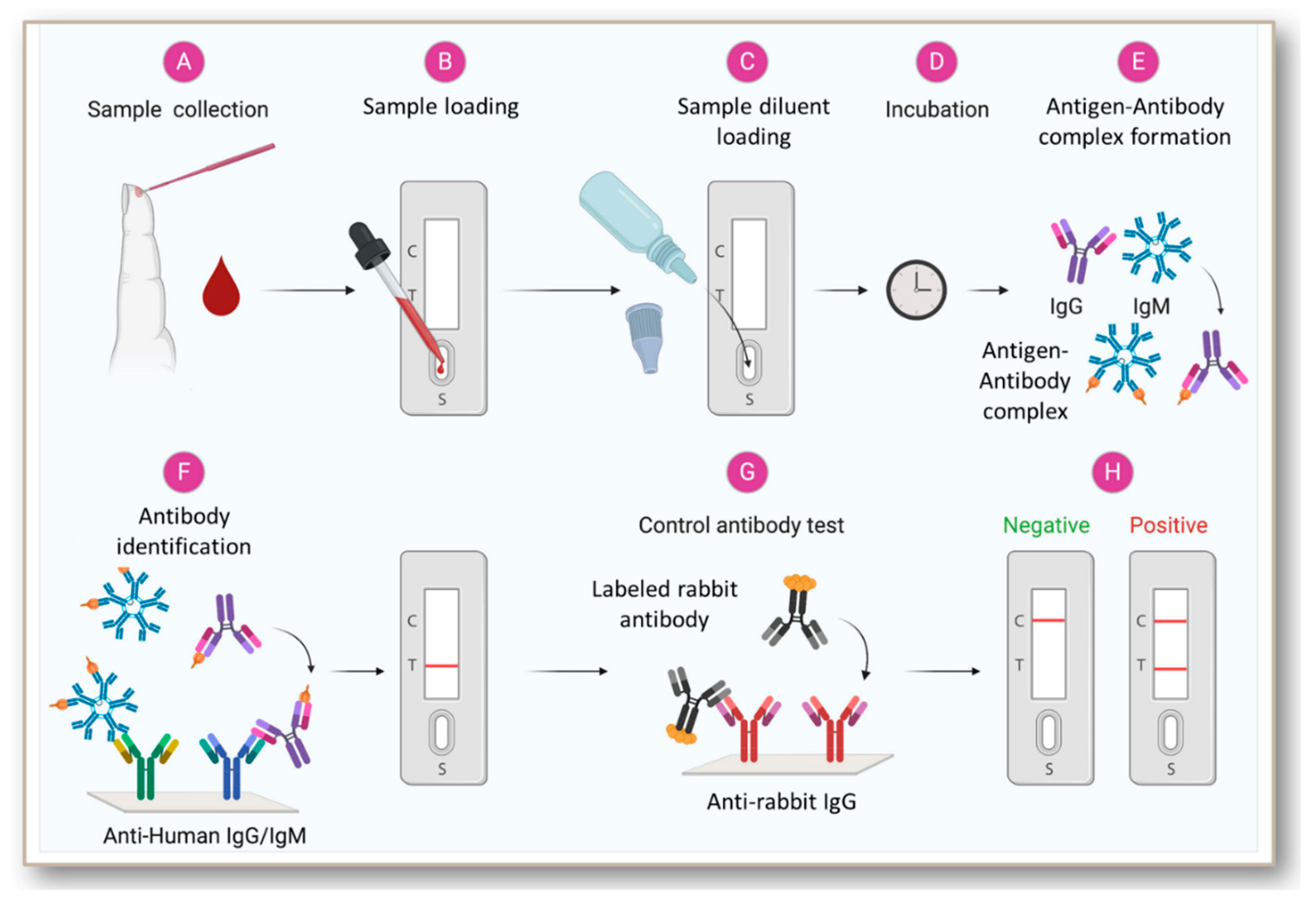
Jcm Free Full Text Rapid Antibody Based Covid 19 Mass Surveillance Relevance Challenges And Prospects In A Pandemic And Post Pandemic World Html

Coronavirus Igg Igm Iga Antibody Test Available Allcare Allcare Family Medicine Urgent Care
Posting Komentar untuk "What Covid 19 Antibody Tests Are Fda Approved"